Electrolysis is a generic term in chemistry, which covers all reactions triggered, intensified or modified by the application of current. The reactions in a battery are just as much a part of this as the galvanic processes that this dictionary usually deals with.
Electrolysis in the electroplating sense is understood to mean the redox reactions produced under the influence of the electric current, which lead to the chemical conversion of the electrolyte ingredients and the electrode materials.
The electrolytic dissolution of metals or the deposition from an aqueous medium for cleaning or layer generation is also usually referred to as electrolysis here.
This includes:
the catalytic metal deposition
the electrolytic refining of e.g. copper
charging an accumulator
the separation of aluminium metal from a bauxite salt melt
Electrolysis is the reversal of the processes in a battery, the discharge of an accumulator or the operation of a fuel cell.
In electrolysis, electrical energy is converted into chemical energy.
This is also the aim of electrolysis, especially when water is broken down into hydrogen and oxygen.
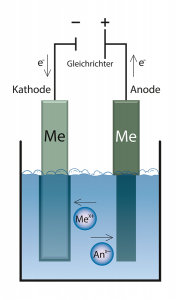
An electric direct current is conducted through two electrodes through a conductive liquid (electrolyte). At the electrodes, the electrolysis produces reaction products from the substances contained in the electrolyte.
The voltage source causes a lack of electrons in the electrode connected to the positive pole (anode) and an excess of electrons in the other electrode connected to the negative pole (cathode). The solution (electrolyte) between the cathode and anode contains positively and negatively charged ions as charge carriers. The positively charged cations migrate to the negatively charged cathode by applying a voltage. At the cathode they take up one or more electrons and are thus reduced. The opposite process takes place at the anode. There the negatively charged anions give off electrons, i.e. they are oxidised. With soluble anodes, the metal is oxidized to the ionic form by the release of electrons and thus goes into solution. The amount of electrons transferred at the anode is equal to the amount transferred at the cathode.
An electric direct current is conducted through two electrodes through a conductive liquid (electrolyte). At the electrodes, the electrolysis produces reaction products from the substances contained in the electrolyte.
The voltage source causes a lack of electrons in the electrode connected to the positive pole (anode) and an excess of electrons in the other electrode connected to the negative pole (cathode). The solution (electrolyte) between the cathode and anode contains positively and negatively charged ions as charge carriers. The positively charged cations migrate to the negatively charged cathode by applying a voltage. At the cathode they take up one or more electrons and are thus reduced. The opposite process takes place at the anode. There the negatively charged anions give off electrons, i.e. they are oxidised. With soluble anodes, the metal is oxidized to the ionic form by the release of electrons and thus goes into solution. The amount of electrons transferred at the anode is equal to the amount transferred at the cathode.
The minimum voltage that must be applied for electrolysis is called the decomposition voltage (Uz or Ez). This or a higher voltage must be applied for electrolysis to take place at all. If this minimum voltage is not exceeded, an insulating double layer inhibits the electrochemical conversion reactions.
For each substance, for each conversion of ions to two-atomic or multi-atomic molecules, the decomposition voltage, the deposition potential can be determined from the redox potential. From the redox potential one receives further information, such as that for the electrolytic decomposition of metal electrodes in acid or for the change of the decomposition voltage depending on the pH value. For example, the redox potential can be used to calculate that the anodic oxygen formation during the electrolysis of water in a basic solution (decomposition voltage: 0.401 V) takes place at a lower voltage than in an acidic (decomposition voltage: 1.23 V) or neutral (decomposition voltage: 0.815 V) solution. This is explained by the definition of pH and pOH, according to which a low pH (acidic reaction) corresponds to a high concentration of hydrogen ions (large supply of hydrogen), whereas a high pH corresponds to a high content of OH- ions (basic reaction) (large supply of decomposable OH– ions).
If several reducible cations are present in an electrolyte solution, first those cations are reduced which have a more positive (noble) potential in the redox series (voltage series). During the electrolysis of an aqueous saline solution, hydrogen and not sodium is always formed at the cathode, since the hydrogen ions of water react more nobly than the extremely base sodium. Even if there are several types of anions that can be oxidized, the ones that are as close as possible to the voltage zero in the redox series, i.e. have a weaker positive redox potential, are used first.
After exceeding the decomposition voltage, the current intensity increases proportionally with the voltage increase. According to Faraday, the weight of an electrolytically formed substance is proportional to the electrical charge that has flowed (current intensity multiplied by time, see Faraday’s laws). For the formation of 1 g hydrogen (about 11.2 litres, two electrons are needed to form a hydrogen molecule) from aqueous solution, an electrical charge of 96485 C (1 C = 1 A-s) is required. At a current of 1 A, the formation of 11.2 litres of hydrogen thus takes 26 hours and 48 minutes (=96485 s).
Besides the redox potential, the overvoltage (the overpotential) is also important. Due to kinetic inhibitions at electrodes, a much higher voltage is often required than is calculated from the redox potentials. The overvoltage effects can – depending on the material properties of the electrodes – also change the redox series so that other ions are oxidized or reduced than would have been expected according to the redox potential.
Shortly after switching off an electrolysis, a current surge in the other direction can be detected with an ammeter. In this short phase the reverse process of electrolysis, the formation of a galvanic cell, begins. In this process, electricity is not consumed for the conversion, but electricity is generated for a short time; this principle is used in fuel cells. The cause of this phenomenon in electrolysis cells is the dissolution of the electrode films in the electrolyte. This compensates for the dynamic imbalance of the electrolysis caused by the current flow, which produces a brief potential reversal.
Sometimes it is advisable to separate the cathode compartment and the anode compartment from each other to avoid undesirable chemical reactions and to allow the charge exchange between the anode and cathode compartment to take place only through a porous diaphragm – often an ion exchange resin. This is quite important in technical electrolysis for the production of caustic soda. Knowledge of molar limit conductivities can also be important for tracking the conversion of substances and the migration rates of ions.
If the separation of individual molecules or bonds is forced by electrolysis, a galvanic element is simultaneously effective, the voltage of which may counteract the electrolysis. This voltage is also called polarization voltage.